M&D Clinical Corner: Lyme Disease
The Clinical Corner is a monthly feature that highlights a variety of important pharmacist topics that is written by Dr. Jesica Mills, PharmD, ND, MBA, RPh, BCES, BCLS, BCNP.
- What is Lyme?
- What Co-infections Can Be Present?
- Symptoms and Clinical Manifestations
- Diagnosis and Testing- Current Challenges and Limitations
- Guidelines for Treatment and Management
- Prevention Tips
- Recommendations for Pharmacists
What is Lyme Disease?
- Lyme disease, caused by the spirochete Borrelia burgdorferi and transmitted by Ixodes ticks, is a significant public health concern globally.
- It is responsible for 77% of the vector-borne infections in the US, and can affect every area in the human body, and complicate the immune system.
- The disease is characterized with the initial presentation of the erythema migrans (EM) rash following a tick bite.
- However, Lyme disease can also present with neurological, cardiac, and musculoskeletal symptoms. (García-Moncó & Benach, 2019; Krause et al., 2003).
- To complicate the presentation even more, there are multiple common co-infections that can be present.
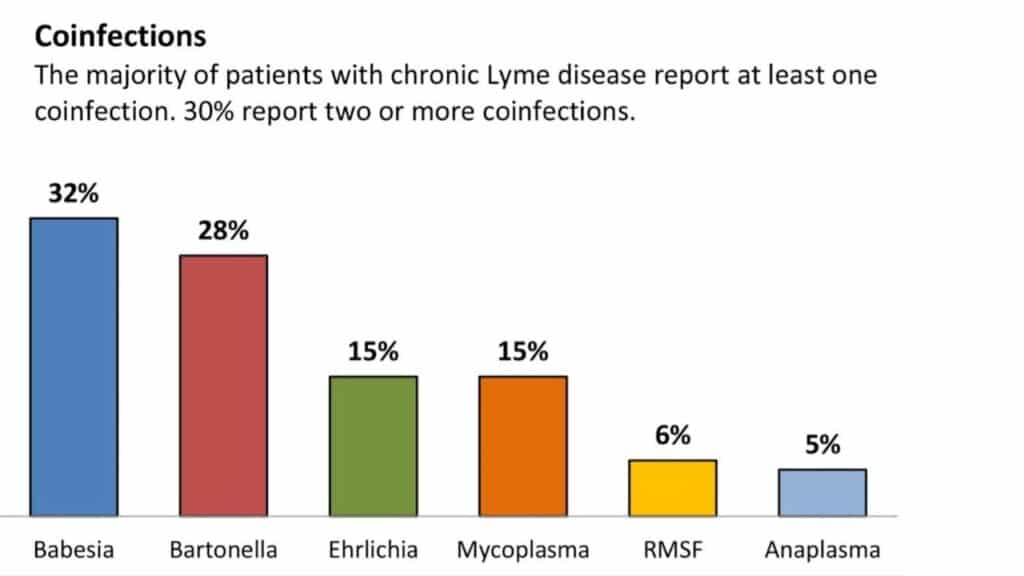
What Co-Infections Can Be Present?
- A study found that 18.7% of patients tested positive for co-infection with both Borrelia burgdorferi and Babesia microti, a common co-infection (Primus et al., 2018). Showing the importance of considering multiple pathogens in the diagnosis and management of Lyme disease cases, and utilizing different labs to test for multiple infectious agents.
- Studies show the bacteria can lie dormant and become persistent and hard to treat when not in the acute phase.
- In vectors- ticks, mosquitoes, fleas, spiders, etc- they can transmit diseases from animals like mice, squirrels, and rabbits when they bite animals then humans. Ticks carry many bacteria, viruses, fungi, and protozoans and can transmit these in a single bite.
- The most common co-infections that patients present with are bartonella (cat scratch fever and trench fever), babesiosis, anaplasmosis, ehrlichiosis, relapsing fever, tularemia, and Rocky Mountain Spotted Fever (RMSF).
- Source: https://www.lymedisease.org/lyme-basics/co-infections/about-co-infections/
Symptoms and Clinical Manifestations
- Early Localized Phase:
- Presents from 3-30 days after a tick bite.
- The EM rash appears in 80% of patients (not all patients), and general symptoms of fatigue, malaise, and fever, similar to the flu.
- Early Disseminated Phase:
- Days to weeks after a tick bite, when the bacteria has gotten into the circulation. Patients can present with general symptoms of lymphadenopathy, skin manifestations, migratory joint and muscle pain, meningitis, and neurological and cardiac symptoms.
- Neurological:
- Headache, stiff neck, pain and tingling in the extremities, Bell’s Palsy, mood disorders, memory deficits, sleep issues are all observed.
- Cardiac:
- myopericarditis with left ventricular dysfunction and mitral regurgitation, typically occurring about 1 to 2 months after infection (Malik et al., 2021). Lyme carditis (AV block) is also present in 1% of cases.
- Late Disseminated Phase:
- Typically presents with intermittent swelling and pain in one or more joints, especially affecting large joints. This manifestation usually occurs months to years (6-36 months) after the initial infection. (“Garin-Bujadoux-Bannwarth Meningoradiculoneuritis: A Case Report”, 2022).
- Late disseminated Lyme disease can also cause neurologic and cardiac manifestations similar to those seen in early disease stages.
- Symptoms can range depending on the co-infections present, and can be more severe and cause a longer recovery.
- Babesiosis often presents with flu-like symptoms that can be challenging to differentiate from other conditions (Wormser et al., 2019; Aliota et al., 2014).
- Anaplasmosis: Caused by Anaplasma phagocytophilum, leading to fever, headache, and muscle aches (Berghoff, 2012).
- Powassan virus: A rare but severe infection transmitted by Ixodes ticks, causing encephalitis (Berghoff, 2012).
- Ehrlichiosis manifests as fever, headache, and muscle aches (Berghoff, 2012).
- Bartonellosis leads to a wide range of symptoms including fever, fatigue, and neurological manifestations.
- Tularemia caused by Francisella tularensis, presents with a fever, skin ulcers, and swollen lymph nodes.
- Rocky Mountain Spotted Fever is caused by Rickettsia rickettsii, resulting in fever, rash, and headache.
Diagnosis and Testing- Current Challenges and Limitations
- Diagnosing Lyme disease presents several challenges and limitations due to the complexities involved in clinical diagnosis, serologic testing interpretation, and conflicting guidelines for diagnosis and treatment.
- The diagnosis of Lyme disease typically relies on a combination of clinical findings, a history of exposure in an endemic area, and relevant serologic testing to support clinical observations Miraglia (2016).
- The current Centers for Disease Control and Prevention recommends a two-tiered algorithm for serodiagnosis, the initial enzyme immunoassay (EIA) followed by separate IgM and IgG Western blots if the EIA result is positive or borderline Marques (2015). A positive test requires you to have at least 5 or more antibodies out of 10, or else it is considered negative.
- False-negative results in EIA testing can occur, leading to potential underdiagnosis of Lyme disease. This can be attributed to the genetic diversity of Borrelia burgdorferi, which may not be fully captured in two-tiered testing, potentially resulting in missed cases (Wormser et al., 2013).
- Failure to recognize erythema migrans or atypical presentations without a rash can lead to missed or delayed diagnoses, ineffective antibiotic treatment, and the potential for late disease manifestations (Aucott et al., 2009). The lack of recognition of chronic Lyme disease by the Infectious Diseases Society of America (IDSA) contributes to underdiagnosis.
- The diagnostic ambiguity between Lyme arthritis and septic arthritis can complicate management decisions (Tout et al., 2021).
- Addressing these limitations through improved testing strategies and consensus on the recognition of chronic Lyme disease is crucial for enhancing diagnostic accuracy and ensuring appropriate management of Lyme disease.
- Polymerase chain reaction (PCR) testing is a valuable tool. PCR testing directly detects the genetic material of Borrelia burgdorferi in patient samples. This method offers a more direct and sensitive approach to identifying the presence of the pathogen, especially in the early stages of infection when antibodies may not be detectable (Wormser & Schwartz, 2009, as well as in cases where patients continue to exhibit symptoms post-treatment.
- Studies have demonstrated the presence of Borrelia burgdorferi DNA through PCR testing in animals even after antibiotic treatment, highlighting the potential of PCR to detect persistent infection that may not be captured by culture-based methods (Wormser & Schwartz, 2009).
- The Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire (HMQ) has been validated as a valuable tool for assessing Lyme disease symptoms Horowitz & Freeman (2019). It contains specific symptoms such as migratory joint pain, muscle pain, and nerve pain. These have been associated with Lyme disease and are not commonly found in other overlapping conditions like fibromyalgia (Citera et al., 2017).
- This questionnaire has been instrumental in differentiating Lyme disease symptoms from those of other illnesses, aiding in the empirical validation of suspected Lyme disease cases (Citera et al., 2017). By analyzing patient responses to the questionnaire, clinicians can gain insights into the symptomatology and severity experienced by individuals with chronic Lyme disease or post-treatment Lyme disease syndrome (PTLDS) (Horowitz & Freeman, 2018).
Guidelines for Treatment and Management
- There are 2 sets of guidelines for treatment, IDSA and ILADS, and they differ significantly in the duration of antibiotic therapy. These differences highlight the complexity and variability in managing Lyme disease and underscore the importance of considering multiple perspectives in clinical decision-making.
- IDSA:https://academic.oup.com/cid/article/72/1/e1/6010652
- Early Localized Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 10-21 days
- Early Disseminated Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 14-28 days
- Late Disseminated Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 14-28 days
- Late Lyme disease with arthritis is typically treated with oral antibiotics such as doxycycline, amoxicillin, or cefuroxime, and intravenous ceftriaxone may be necessary for nervous system involvement (Sharma, 2018).
- ILADS:https://www.ilads.org/patient-care/ilads-treatment-guidelines/
- Early Localized Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 14-21 days
- Early Disseminated Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 21-28 days
- Late Disseminated Lyme Disease:
- Drug: Doxycycline, Amoxicillin, or Cefuroxime
- Duration: 28-42 days
- ILADS recommends against the use of a single 200 mg dose of doxycycline for the prevention of Lyme disease.
Based on animal studies, ILADS recommends that known blacklegged tick bites be treated with 20 days of doxycycline (barring any contraindications). - Given the low success rates in trials treating EM rashes for 20 or fewer days, ILADS recommends that patients receive 4-6 weeks of doxycycline, amoxicillin or cefuroxime. A minimum of 21 days of azithromycin is also acceptable, especially in Europe. All patients should be reassessed at the end of their initial therapy and, when necessary, antibiotic therapy should be extended.
Prevention Tips
- Lyme disease has been found in all 50 states, but there is still the misconception that it is only present in the northeast US. Prevention is needed for all no matter the location.
- In the US, the CDC says it’s only in the Ixodes tick, and it must be attached for 36-48 hours or more in order for a patient to contract Lyme. In Europe, there are over 5 types of ticks that are thought to cause Lyme disease, and in a shorter amount of time. Due to the range in symptoms, limitations and variance in diagnosis, false-negative rates of testing, and the multiple guidelines for treatment, avoiding ticks is the best strategy.
- Personal protective measures: Wearing appropriate clothing, using insect repellents, and removing ticks promptly can reduce exposure to ticks (Ogden et al., 2015). DEET is recommended, and has some documented hazardous side-effects possible. Most Lyme-Literate professionals recommend using Picaridin.
- Environmental risk reduction: Controlling ticks and tick infections through pesticide applications can help reduce the environmental risk of Lyme disease transmission. Modifying habitats to reduce tick populations and minimize human-tick interactions. (Finch et al., 2014).
- Tick avoidance: Avoiding areas where ticks that transmit Lyme disease are active, especially during peak tick activity times, can lower the risk of tick bites and subsequent infection.
- Tick checks: Conducting daily tick checks and promptly removing any attached ticks (Ogden et al., 2015). Ensure to remove the entire tick, and utilize tools such as a tick-twister to prevent any pieces from remaining.
- Antibiotic prophylaxis: In cases of recognized tick bites with a high risk of Borrelia burgdorferi infection, a single dose of doxycycline within 72 hours post-bite can prevent the development of Lyme disease (Nadelman et al., 2001).There are studies showing the topical use of azithromycin within the above timeframe was effective at killing the bacteria causing Lyme.
Recommendations for Pharmacists
- Recognize the signs and symptoms of Lyme in the various stages, and realize there are testing limitations and the possibility of a missed diagnosis.
- Consider offering access to ordering PCR testing for those who have exposure to tick bites, and have multiple symptoms. You can use the Horowitz Scale or HMQ to determine the risk of Lyme based on their symptoms. (https://projectlyme.org/msids-questionnaire/)
- If not treated, the disease can progress to early disseminated disease with cardiac and neurologic involvement, and eventually to late disease with manifestations such as arthritis.Collaborate with healthcare providers to ensure appropriate antibiotic therapy for known tick bites and erythema migrans rashes, as well as retreatment for persistent manifestations of Lyme disease.
- Educate patients on prevention, how to remove a tick and making the appropriate tools for removal available (https://www.otom.com/en/). Educate on the importance of getting tested at the right time.
- The best time to be tested for Lyme disease after a tick bite is typically around 2-4 weeks post-bite. This allows the body to develop detectable levels of antibodies against Borrelia burgdorferi. Testing too soon may result in false-negative results due to insufficient time for the immune response to produce detectable antibodies. Conversely, testing too long after a tick bite may delay diagnosis and treatment.
- Answer their questions about when and if they should see a provider:
- After removing a tick, consider seeking medical attention if signs and symptoms of early Lyme disease occur (a rash or flu-like symptoms).
- If these symptoms manifest after a tick bite, it is advisable to consult a healthcare provider promptly for evaluation.
- If the tick has been attached for more than 36 hours, antibiotic prophylaxis should be considered within 72 hours of tick removal to prevent the development of Lyme disease (Clark & Hu, 2008). Immediate removal of attached ticks can also help prevent Lyme disease infection (Clark & Hu, 2008).
- Remember that Lyme Disease is a CDC Reportable Disease, which means cases need to be reported to your local health department. This is done by sending in the Reportable Disease Form in your state. Contact your health department to get this form if needed, and ask your patient if their case has been reported.
- When determining the treatment regimen for a patient with Lyme, remember there are 2 sets of guidelines- IDSA and ILADS.
References:
Garcia-Monco JC, Benach JL. Lyme Neuroborreliosis: Clinical Outcomes, Controversy, Pathogenesis, and Polymicrobial Infections. Ann Neurol. 2019 Jan;85(1):21-31. doi: 10.1002/ana.25389. PMID: 30536421; PMCID: PMC7025284.
Krause PJ, Telford SR 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996 Jun 5;275(21):1657-60. PMID: 8637139.
Djokic V, Akoolo L, Primus S, Schlachter S, Kelly K, Bhanot P, Parveen N. Protozoan Parasite Babesia microti Subverts Adaptive Immunity and Enhances Lyme Disease Severity. Front Microbiol. 2019 Jul 10;10:1596. doi: 10.3389/fmicb.2019.01596. PMID: 31354683; PMCID: PMC6635642.
Malik MB, Baluch A, Adhikari S, Quraeshi S, Rao S. Early Onset Lyme Myopericarditis With Left Ventricular Dysfunction and Mitral Regurgitation. J Investig Med High Impact Case Rep. 2021 Jan-Dec;9:23247096211045267. doi: 10.1177/23247096211045267. PMID: 34541925; PMCID: PMC8458661.
(2022). Garin-bujadoux-bannwarth meningoradiculoneuritis: a case report. Annals of Case Reports, (5). https://doi.org/10.29011/2574-7754.100933
Guérin M, Shawky M, Zedan A, Octave S, Avalle B, Maffucci I, Padiolleau-Lefèvre S. Lyme borreliosis diagnosis: state of the art of improvements and innovations. BMC Microbiol. 2023 Aug 1;23(1):204. doi: 10.1186/s12866-023-02935-5. PMID: 37528399; PMCID: PMC10392007.
Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol. 2015 Jun;40(1):198-201. doi: 10.1111/jvec.12153. PMID: 26047204; PMCID: PMC4640675.
Berghoff W. Chronic Lyme Disease and Co-infections: Differential Diagnosis. Open Neurol J. 2012;6:158-78. doi: 10.2174/1874205X01206010158. Epub 2012 Dec 28. PMID: 23400696; PMCID: PMC3565243.
Miraglia, C. M. (2016). A review of the centers for disease control and prevention’s guidelines for the clinical laboratory diagnosis of lyme disease. Journal of Chiropractic Medicine, 15(4), 272-280. https://doi.org/10.1016/j.jcm.2016.08.003
Aucott, J. N., Morrison, C., Muñoz, B., Rowe, P. C., Schwarzwalder, A., & West, S. K. (2009). Diagnostic challenges of early lyme disease: lessons from a community case series. BMC Infectious Diseases, 9(1). https://doi.org/10.1186/1471-2334-9-79
Diuk‐Wasser et al. (2012): Diuk‐Wasser, M. A., Vourc’h, G., Cislo, P., Hoen, A. G., Melton, F., Hamer, S. A., & Fish, D. (2012). Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. American Journal of Tropical Medicine and Hygiene, 86(2), 320-327. doi:10.4269/ajtmh.2012.11-0395
Murray, T. S. and Shapiro, E. D. (2010). Lyme disease. Clinics in Laboratory Medicine, 30(1), 311-328. https://doi.org/10.1016/j.cll.2010.01.003
Sharma, U. (2018). Disseminated lyme disease presenting as multiple non-target cellulitic-appearing skin lesions and oral pseudomembrane. BMJ Case Reports, bcr-2018-225921. https://doi.org/10.1136/bcr-2018-225921
Tout, A. R., McClincy, M. P., Anderson, A., Nowalk, A., & Campfield, B. T. (2021). The impact of operative intervention in pediatric lyme arthritis. Journal of Pediatric Orthopaedics, 41(10), e911-e916. https://doi.org/10.1097/bpo.0000000000001959
Wormser, G. P. and Schwartz, I. (2009). Antibiotic treatment of animals infected withborrelia burgdorferi. Clinical Microbiology Reviews, 22(3), 387-395. https://doi.org/10.1128/cmr.00004-09
Citera, M., Freeman, P. R., & Horowitz, R. (2017). Empirical validation of the horowitz multiple systemic infectious disease syndrome questionnaire for suspected lyme disease. International Journal of General Medicine, Volume 10, 249-273. https://doi.org/10.2147/ijgm.s140224
Horowitz, R. and Freeman, P. R. (2018). Precision medicine: the role of the msids model in defining, diagnosing, and treating chronic lyme disease/post treatment lyme disease syndrome and other chronic illness: part 2. Healthcare, 6(4), 129. https://doi.org/10.3390/healthcare6040129



