November 16, 2023
M&D Clinical Corner: Community Pharmacist’s Guide to COVID-19 mRNA Vaccine Updates
The Clinical Corner is a monthly feature that highlights a variety of important pharmacist topics that is written by Morris & Dickson’s staff pharmacist, Paula Belle (RPh).
This month’s Clinical Corner will discuss recent changes to mRNA COVID-19 vaccinations and processes.
- General Information
- CDC Recommendations
- CDC Interim Clinical Considerations for the Use of COVID-19 Vaccines
- 2023 COVID-19 Booster Vaccination Rollout
- Resources for Pharmacists
GENERAL INFORMATION
- COVID-19 burden is currently lower than at previous points in the pandemic; however, the absolute number of hospitalizations and deaths remains high. [1]
- Last fall and winter virus season, people who received the 2022-2023 COVID-19 vaccine had greater protection against severe illness and hospitalization than those who did not receive that vaccine. [1]
- We are still at risk of COVID-19 since the virus continues to change and new variants emerge. [1]
- Additionally, protection from COVID-19 vaccines and infection decline over time. [1]
- An updated COVID-19 vaccine provides enhanced protection against the variants currently responsible for most hospitalizations in the United States. [1]
- The benefits of COVID-19 vaccination continue to outweigh any potential risks. Serious reactions after COVID-19 vaccination are rare. [1]
- In one study, the risk of cardiac complications, including myocarditis, in males 12-17 years old was 1.8 – 5.6 times higher after COVID-19 infection than after COVID-19 vaccination. [1]
- The vaccines are covered by insurance, including private insurance, Medicare plans, and Medicaid plans. Uninsured children and uninsured adults also have access through the Vaccine for Children Program and Bridge Access Program, respectively. [1]
CDC RECOMMENDATIONS
- On September 11, 2023, the U.S. Food and Drug Administration took action approving and authorizing for emergency use updated COVID-19 vaccines formulated to more closely target currently circulating variants and to provide better protection against serious consequences of COVID-19, including hospitalization and death. [2]
- The September 11, 2023 actions relate to updated mRNA vaccines for 2023-2024 manufactured by ModernaTX Inc. and Pfizer Inc. [2]
- On Tuesday September 12, 2023 the Centers for Disease Control and Prevention (CDC) issued a press release containing new recommendations for COVID-19 Vaccination. [3]
- CDC recommends everyone 6 months and older get an updated COVID-19 vaccine to protect against the potentially serious outcomes of COVID-19 illness this fall and winter. [3]
- Updated COVID-19 vaccines from Pfizer-BioNTech and Moderna were made available during the week of September, 12, 2023. [3]
CDC INTERIM CLINICAL CONSIDERATIONS FOR THE USE OF COVID-19 VACCINES
- An infographic from CDC that details COVID-19 2023-2024 vaccination recommendations for most patients is seen below.
- A printable PDF of this CDC infographic is available at: 2023-2024 COVID-19 CDC Vaccination Recommendations
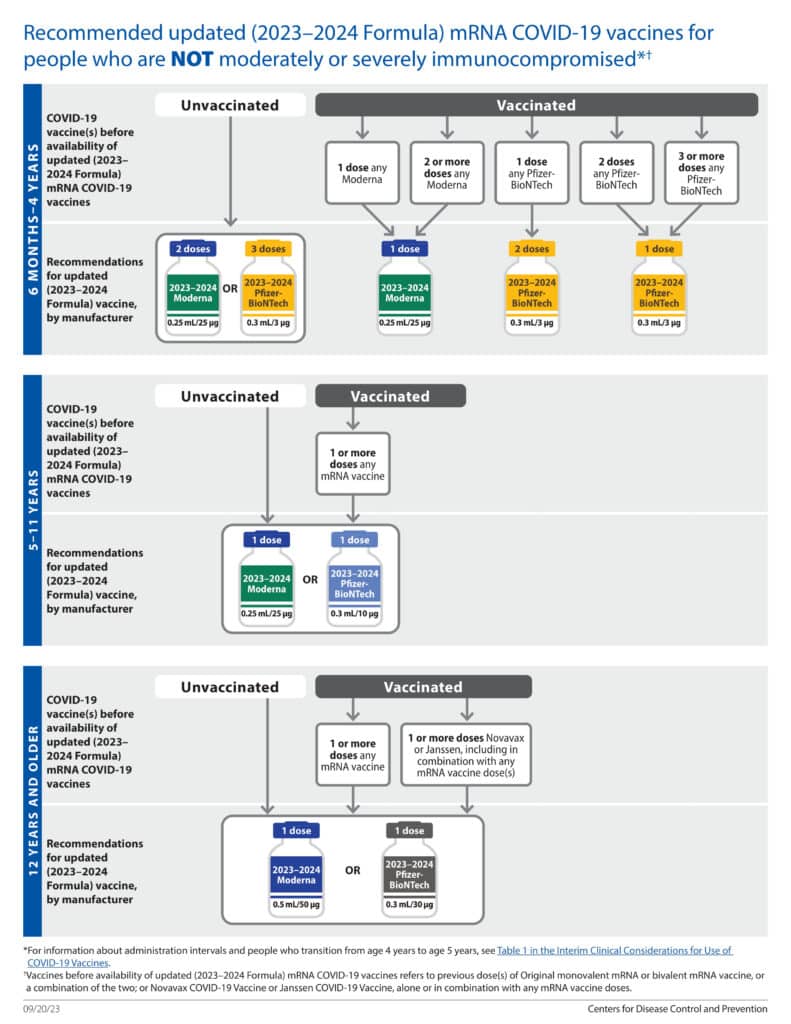
- Everyone ages 5 years and older is recommended to receive 1 dose of updated (2023–¬2024 Formula) mRNA COVID-19 vaccine. [4]
- Children ages 6 months–4 years [4] :
- Initial vaccination: should receive either 2 doses of updated (2023-2024 Formula) Moderna or 3 doses of updated (2023-2024 Formula) Pfizer-BioNTech COVID-19 vaccine.
- Received previous mRNA doses: need 1 or 2 doses of updated (2023-2024 Formula) Moderna or updated (2023-2024 Formula) Pfizer-BioNTech COVID-19 vaccine, depending on the number of prior doses.
- People who are moderately or severely immunocompromised [4] :
- Initial vaccination: should receive a 3-dose series of updated (2023-2024 Formula) Moderna or updated (2023-2024 Formula) Pfizer-BioNTech COVID-19 vaccine.
- Received previous mRNA doses: need 1 or 2 doses of updated (2023-2024 Formula) Moderna or updated (2023–¬2024 Formula) Pfizer-BioNTech COVID-19 vaccine, depending on the number of prior doses.
- May receive 1 or more additional updated (2023-2024 Formula) mRNA COVID-19 vaccine doses.
- Bivalent mRNA COVID-19 vaccines are no longer recommended in the United States. [4]
- In accordance with General Best Practice Guidelines for Immunization, routine administration of all age-appropriate doses of vaccines simultaneously, also known as coadministration, is recommended for children, adolescents, and adults if there are no contraindications at the time of the healthcare visit. [4]
- Simultaneous administration is defined as administering more than one vaccine on the same clinic day, at different anatomic sites, and not combined in the same syringe. [4]
- Providers may simultaneously administer COVID-19, influenza, and respiratory syncytial virus (RSV) vaccines to eligible patients. [4]
- People, particularly adolescent or young adult males, who are recommended to receive both mpox and COVID-19 vaccines might consider waiting 4 weeks between vaccines. [4]
- This is because of the observed risk for myocarditis and pericarditis after receipt of ACAM2000 orthopoxvirus vaccine and COVID-19 vaccines, and the hypothetical risk for myocarditis and pericarditis after JYNNEOS vaccine. [4]
- However, if a patient’s risk for mpox or severe disease due to COVID-19 is increased, administration of mpox and COVID-19 vaccines should not be delayed. [4]
2023 COVID-19 BOOSTER VACCINE ROLLOUT
- With the end of the Covid-19 public health emergency in the spring, the Covid-19 vaccine market has transitioned to the private market. [5]
- But while everyone is entitled to a free Covid-19 shot, the shots aren’t free for every person at every location. People looking for a vaccine through the Bridge program, for example, may have to search to find a provider participating in the program. People with private insurance may find they need to get their shot through their primary care physician, or through one pharmacy, but not another. [5]
- Some insurers have been slow to start paying for Covid-19 shots, creating more challenges. [5]
- There’s a process in the insurance industry known as “turning on the codes” — programing a company’s billing system to recognize and accept claims for Covid-19 shots — that each insurer must go through. Some companies are quicker to do it than others. [5]
- The Department of Health and Human Services (DHH) knows this has been a problem, even though it has been working with insurers and pharmacists for over a year to try to ensure a smooth transition to private market delivery of Covid-19 vaccine. [5]
- On hearing of problems patients have been encountering, the Centers for Medicare and Medicare Services (CMS) recently began to reach out to insurers to remind them that they are obliged to cover the cost of Covid-19 shots for their enrollees. [5]
- CDC’s Bridge Access Program provides no-cost COVID-19 vaccines to adults without health insurance and adults whose insurance does not cover all COVID-19 vaccine costs. No-cost COVID-19 vaccines through this program will be available until December 31, 2024. [6]

RESOURCES FOR PHARMACISTS
General COVID-19 Vaccination Resources
- Updated (9-15-2023) CDC COVID-19 Vaccine Recommendations are available at: CDC Updated COVID-19 Vaccine Recommendations
- A COVID-19 Vaccination Schedule for patients who are NOT moderately or severely immunocompromised is available at: COVID-19 Vaccination Schedule for NON-immunocompromised Patients
- A COVID-19 Vaccination Schedule for patients who ARE immunocompromised is available at: COVID-19 Vaccination Schedule for Immunocompromised Patients
- Details on the CDC’s Bridge Access Program can be obtained at: CDC BRIDGE ACCESS PROGRAM
PFIZER
- Information on the 2023-2024 formula of Pfizer-BioNTech COVID-19 Vaccine is available at : PFIZER-BioNTech COVID-19 Vaccine
- Recipient and caregiver fact sheets for 2023-2024 Pfizer-BioNTech COVID-19 Vaccine are available at: 2023-2024 PFIZER-BioNTech COVID-19 Vaccine Recipient Fact Sheet
- Healthcare provider (HCP) fact sheet for 2023-2024 Pfizer-BioNTech COVID-19 Vaccine is available at: 2023-2024 PFIZER-BioNTech COVID-19 Vaccine HCP Fact Sheet
MODERNA
- Information on the 2023-2024 formula of Moderna COVID-19 Vaccine is available at: MODERNA COVID-19 Vaccine
- Recipient and caregiver fact sheets for 2023-2024 Moderna COVID-19 Vaccine are available at: 2023-2024 MODERNA COVID-19 Vaccine Recipient Fact Sheet
- Healthcare provider (HCP) fact sheet for 2023-2024 Moderna COVID-19 Vaccine is available at: 2023-2024 MODERNA COVID-19 HCP Fact Sheet
Sources
- Centers for Disease Control and Prevention. Updated COVID-19 Vaccine Recommendations Now Available | CDC. 2023 2023-09-13T10:08:34Z [cited 2023 September]; Available from: https://www.cdc.gov/respiratory-viruses/whats-new/covid-vaccine-recommendations-9-12-2023.html.
- U.S. Food and Drug Administration. FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants | FDA. 2023 Mon, 09/11/2023 – 13:00 [cited 2023 September]; Available from: https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating.
- Centers for Disease Control and Prevention. CDC Recommends Updated COVID-19 Vaccine for Fall/Winter Virus Season | CDC Online Newsroom | CDC. 2023 2023-09-14T04:04:08Z [cited 2023 September]; Available from: https://www.cdc.gov/media/releases/2023/p0912-COVID-19-Vaccine.html.
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC. 2023 2023-09-15T09:50:28Z [cited 2023 September]; Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
- Branswell, H. Rollout of Covid vaccines is bumpy, but not unexpected, experts say. 2023 2023-09-26 [cited 2023 September]; Available from: https://www.statnews.com/2023/09/26/rollout-of-covid-vaccines-is-bumpy-but-not-unexpected-experts-say/.
- Centers for Disease Control and Prevention. Bridge Access Program | CDC. 2023 2023-09-27T04:58:57Z; Available from: https://www.cdc.gov/vaccines/programs/bridge/index.html.



